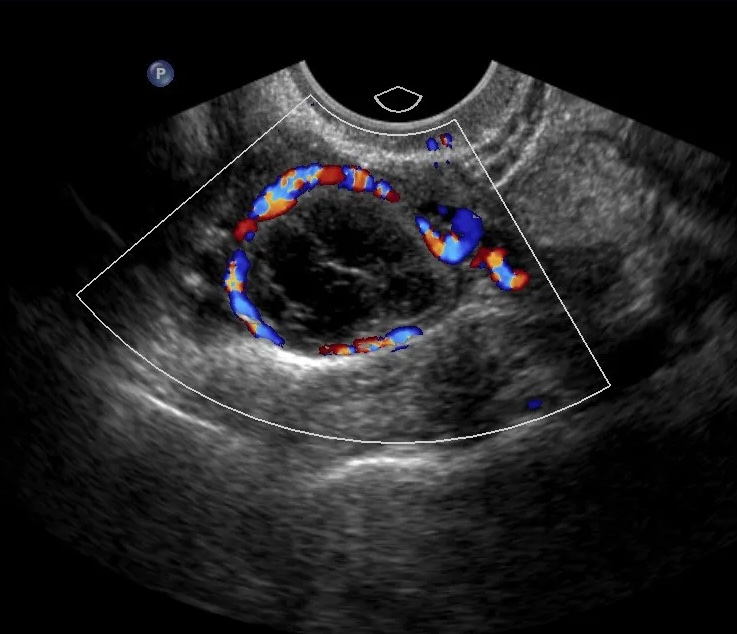
The diagnostic gap isn't experienced equally


Black and Indigenous American patients are 23% less likely to receive ovarian cancer diagnosis with tools validated using 80-98% White populations7.
Financial toxicity affects 47% of gynecologic cancer patients. Only 39% of patients receive recommended testing, varying by insurance status8.
99% of gynecologic oncologists work in metropolitan counties, with only 13% practicing in areas with populations <50,0009.